
About PneumaCare
PneumaCare Ltd is a Cambridge based company, pioneers of the technology – Structured Light Plethysmography (SLP). Our revolutionary, respiratory imaging system provides assessment solutions, that are non-contact, non-invasive and non-aerosol generating. Breaking new ground, PneumaCare Ltd are at the forefront in changing the face of assessment in the respiratory field.
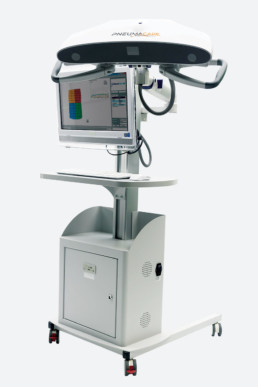
PneumaCare has received product approvals including CE marking and FDA 510(k) for its Thora-3Di® product.
Founded in 2009, the company’s initial focus was centred on the clinical need for better lung function assessment in children.
Advancement in Motion Capture and CGI for animation provide the initial ideas for PneumaCare’s SLP technologies. Using the concept of marker motion tracking, PneumaCare’s engineering team developed the novel ability to track movement in patients without physical markers.
Since that time, PneumaCare has expanded this focus to include adults in the lung function, ICU and thoracic surgery segments. PneumaCare markets Thora-3Di®, an imaging and assessment device utilising novel non-contact 3D-imaging technology. This breakthrough in patient care is presently operating in a range of hospital environments from paediatrics through to surgical assessment and adult intensive care.
A Message from our CEO

Bart A. Lewin
Chief Executive Officer
“PneumaCare® has become even more confident that our Thora-3Di® device, which provides non-contact, non-invasive, and non-aerosol generating lung function assessment, has more relevance than ever before.
“Despite delays caused by the pandemic, our COPD clinical trial has progressed significantly, hopefully clearing a path as a device to aid clinicians in diagnosing and establishing treatment paths for COPD—a devastating disease causing suffering throughout the UK.
“PneumaCare® has also engaged in engineering efforts leading to upgrades in technology; incorporating exciting new features based on learnings and input from loyal clinical supporters and experiences gained from the clinical trial.
“PneumaCare® has established itself as a leader in using SLP technology for lung function assessment, and as our COPD clinical trial progresses, confidently look forward to 2022 and 2023 as pivotal years for the company.”
Appointed as PneumaCare’s Interim Chief Executive Officer in March 2020, Bart Lewin has strengthened and led the company to its next stage of growth. Mr. Lewin has founded six start up technology companies and has co-founded another start-up venture, which invests in and promotes early-stage ventures. Bart has over three decades of business growth management and demonstrated this throughout his career.
Bart has also held several high-profile executive roles including Chief Executive & Technology Officer at NEWave, Inc. and Chief Technology Officer for Pinnacle Entertainment, Inc.
Bart holds a BA in Economics from Reed College, USA. He is a published author, a sought-after public speaker, and a named inventor on multiple International patents.

Careers
At Pneumacare, our employees do work that matters, applying our technology in ways that make a positive impact on treatment pathways. Here you’ll be a part of a company known for uncompromising integrity. We are always looking for dedicated people to join us on our mission to make a difference.
We strive for an inclusive environment where employees feel safe, engaged, valued and free to create and contribute their ideas, driving our company forward.
If you are interested in joining our team email us at hr@pneumacare.com